Chlorophyll (also chlorophyl) is a green pigment found in almost all plants, algae, and cyanobacteria. Its name is derived from the Greek words chloros("green") and phyllon ("leaf"). Chlorophyll is an extremely important biomolecule, critical in photosynthesis, which allows plants to obtain energy from light. Chlorophyll absorbs light most strongly in the blue portion of the electromagnetic spectrum, followed by the red portion. However, it is a poor absorber of green and near-green portions of the spectrum; hence the green color of chlorophyll-containing tissues.[1] Chlorophyll was first isolated by Joseph Bienaimé Caventou and Pierre Joseph Pelletier in 1817.[2]
Contents[hide] |
]Chlorophyll and photosynthesis
Chlorophyll is vital for photosynthesis, which allows plants to obtain energy from light.
Chlorophyll molecules are specifically arranged in and around photosystems that are embedded in the thylakoid membranes of chloroplasts. In these complexes, chlorophyll serves two primary functions. The function of the vast majority of chlorophyll (up to several hundred molecules per photosystem) is to absorb light and transfer that light energy by resonance energy transfer to a specific chlorophyll pair in the reaction center of the photosystems.
The two currently accepted photosystem units are Photosystem II and Photosystem I, which have their own distinct reaction center chlorophylls, named P680 and P700, respectively.[3] These pigments are named after the wavelength (in nanometers) of their red-peak absorption maximum. The identity, function and spectral properties of the types of chlorophyll in each photosystem are distinct and determined by each other and the protein structure surrounding them. Once extracted from the protein into a solvent (such as acetone or methanol),[4][5][6] these chlorophyll pigments can be separated in a simple paper chromatography experiment, and, based on the number of polar groups between chlorophyll a and chlorophyll b, will chemically separate out on the paper.
The function of the reaction center chlorophyll is to use the energy absorbed by and transferred to it from the other chlorophyll pigments in the photosystems to undergo a charge separation, a specific redox reaction in which the chlorophyll donates an electron into a series of molecular intermediates called anelectron transport chain. The charged reaction center chlorophyll (P680+) is then reduced back to its ground state by accepting an electron. In Photosystem II, the electron that reduces P680+ ultimately comes from the oxidation of water into O2 and H+ through several intermediates. This reaction is how photosynthetic organisms like plants produce O2 gas, and is the source for practically all the O2 in Earth's atmosphere. Photosystem I typically works in series with Photosystem II, thus the P700+ of Photosystem I is usually reduced, via many intermediates in the thylakoid membrane, by electrons ultimately from Photosystem II. Electron transfer reactions in the thylakoid membranes are complex, however; and the source of electrons used to reduce P700+ can vary.
The electron flow produced by the reaction center chlorophyll pigments is used to shuttle H+ ions across the thylakoid membrane, setting up achemiosmotic potential used mainly to produce ATP chemical energy; and those electrons ultimately reduce NADP+ to NADPH a universal reductant used to reduce CO2 into sugars as well as for other biosynthetic reductions.
Reaction center chlorophyll-protein complexes are capable of directly absorbing light and performing charge separation events without other chlorophyll pigments, but the absorption cross section (the likelihood of absorbing a photon under a given light intensity) is small. Thus, the remaining chlorophylls in the photosystem and antenna pigment protein complexes associated with the photosystems all cooperatively absorb and funnel light energy to the reaction center. Besides chlorophyll a, there are other pigments, called accessory pigments, which occur in these pigment-protein antenna complexes.
There is as yet no satisfactory scientific explanation as to why chlorophyll has evolved to "ignore" green and near-green light, which are a major part of the visible spectrum.
A green sea slug, Elysia chlorotica, has been found to use the chlorophyll it has eaten to perform photosynthesis for itself; no other animal has been found to have this ability.
]Chemical structure
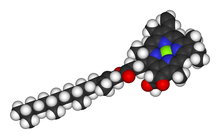
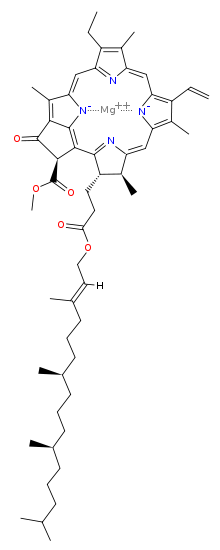
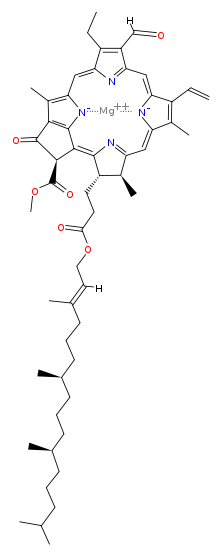
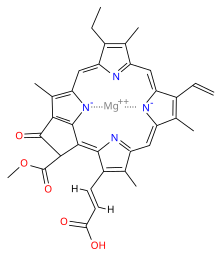
Chlorophyll is a chlorin pigment, which is structurally similar to and produced through the same metabolic pathway as other porphyrin pigments such asheme. At the center of the chlorin ring is a magnesium ion. For the structures depicted in this article, some of the ligands attached to the Mg2+ center are omitted for clarity. The chlorin ring can have several different side chains, usually including a long phytol chain. There are a few different forms that occur naturally, but the most widely distributed form in terrestrial plants is chlorophyll a. The general structure of chlorophyll a was elucidated by Hans Fischer in 1940, and by 1960, when most of the stereochemistry of chlorophyll a was known, Robert Burns Woodward published a total synthesis of the molecule as then known.[7] In 1967, the last remaining stereochemical elucidation was completed by Ian Fleming,[8] and in 1990 Woodward and co-authors published an updated synthesis.[9] In 2010, a near-infrared light photosynthetic pigment called Chlorophyll f may have been discovered in cyanobacteria and other oxygenic microorganisms that form stromatolites.[10][11] Based on NMR data, optical and mass spectra, it is thought to have a structure of C55H70O6N4Mg or [2-formyl]-chlorophyll a.[12]
The different structures of chlorophyll are summarized below:
| Chlorophyll a | Chlorophyll b | Chlorophyll c1 | Chlorophyll c2 | Chlorophyll d | Chlorophyll f | |
|---|---|---|---|---|---|---|
| Molecular formula | C55H72O5N4Mg | C55H70O6N4Mg | C35H30O5N4Mg | C35H28O5N4Mg | C54H70O6N4Mg | C55H70O6N4Mg |
| C2 group | -CH3 | -CH3 | -CH3 | -CH3 | -CH3 | -CHO |
| C3 group | -CH=CH2 | -CH=CH2 | -CH=CH2 | -CH=CH2 | -CHO | -CH=CH2 |
| C7 group | -CH3 | -CHO | -CH3 | -CH3 | -CH3 | -CH3 |
| C8 group | -CH2CH3 | -CH2CH3 | -CH2CH3 | -CH=CH2 | -CH2CH3 | -CH2CH3 |
| C17 group | -CH2CH2COO-Phytyl | -CH2CH2COO-Phytyl | -CH=CHCOOH | -CH=CHCOOH | -CH2CH2COO-Phytyl | -CH2CH2COO-Phytyl |
| C17-C18 bond | Single (chlorin) | Single (chlorin) | Double (porphyrin) | Double (porphyrin) | Single (chlorin) | Single (chlorin) |
| Occurrence | Universal | Mostly plants | Various algae | Various algae | Cyanobacteria | Cyanobacteria |
When leaves degreen in the process of plant senescence, chlorophyll is converted to a group of colourless tetrapyrroles known as nonfluorescent chlorophyll catabolites (NCC's) with the general structure:
These compounds have also been identified in several ripening fruits.[13]
]Spectrophotometry
Measurement of the absorption of light is complicated by the solvent used to extract it from plant material, which affects the values obtained,
- In diethyl ether, chlorophyll a has approximate absorbance maxima of 430 nm and 662 nm, while chlorophyll b has approximate maxima of 453 nm and 642 nm.[14][specify]
- The absorption peaks of chlorophyll a are at 665 nm and 465 nm. Chlorophyll a fluoresces at 673 nm (maximum) and 726 nm. The peak molar absorption coefficient of chlorophyll a exceeds 105 M−1 cm−1, which is among the highest for small-molecule organic compounds.[citation needed]
By measuring chlorophyll fluorescence, plant ecophysiology can be investigated. Chlorophyll fluorometers are used by plant researchers to assess plant stress.]
Biosynthesis
In plants, chlorophyll may be synthesized from succinyl-CoA and glycine, although the immediate precursor to chlorophyll a and b is protochlorophyllide. InAngiosperms, the last step, conversion of protochlorophyllide to chlorophyll, is light-dependent and such plants are pale (etiolated) if grown in the darkness.Non-vascular plants and green algae have an additional light-independent enzyme and grow green in the darkness as well.
Chlorophyll itself is bound to proteins and can transfer the absorbed energy in the required direction. Protochlorophyllide, occurs mostly in the free form, and, under light conditions, acts as photosensitizer, forming highly toxic free radicals. Hence, plants need an efficient mechanism of regulating the amount of chlorophyll precursor. In angiosperms, this is done at the step of aminolevulinic acid (ALA), one of the intermediate compounds in the biosynthesis pathway. Plants that are fed by ALA accumulate high and toxic levels of protochlorophyllide; so do the mutants with the damaged regulatory system.[15]
Chlorosis is a condition in which leaves produce insufficient chlorophyll, turning them yellow. Chlorosis can be caused by a nutrient deficiency including iron - called iron chlorosis, or in a shortage ofmagnesium or nitrogen. Soil pH sometimes play a role in nutrient-caused chlorosis, many plants are adapted to grow in soils with specific pHs and their ability to absorb nutrients from the soil can be dependent on the soil pH.[16] Chlorosis can also be caused by pathogens including viruses, bacteria and fungal infections, or sap-sucking insects.
]Measuring chlorophyll
The chlorophyll content of leaves can be non-destructively measured using hand-held, battery portable optical meters[17]. These meters measure the amount of energy absorbed by the leaf in the red and infrared region of the EMS. Absorption in the red band gives an estimate of the amount of chlorophyll present and the absorption in the infrared band is used to qualify and compensate for varying leaf thickness. The measurements made by these devices are simple, quick and relatively inexpensive. They now, typically, have large data storage capacity, averaging and graphical displays.
Culinary use
Chlorophyll is registered as a food additive (colorant), and its E number is E140. Chefs use chlorophyll to color a variety of foods and beverages green, such as pasta and absinthe.[18] Chlorophyll is not soluble in water and is first mixed with a small quantity of oil to obtain the desired result. Extracted Liquid Chlorophyll was considered unstable and always denatured, until 1997 when Frank S. & Lisa Sagliano used freeze-drying of liquid chlorophyll at the University of Florida and stabilized it as a powder, preserving it for future use.[19]
]See also
- Bacteriochlorophyll, related compounds in phototrophic bacteria
- Chlorophyllin, a semi-synthetic derivative of chlorophyll
- Grow light, a lamp that promotes photosynthesis
- Deep chlorophyll maximum
- Chlorophyll a, an essential chlorophyll pigment
[]References
- ^ Speer, Brian R. (1997). "Photosynthetic Pigments". UCMP Glossary (online). University of California Museum of Paleontology. Retrieved 2010-07-17.
- ^ (September 1951). "Joseph Pelletier and Joseph Caventou". Journal of Chemical Education 28 (9): 454. doi:10.1021/ed028p454. ISSN 0021-9584.
- ^ Green, 1984
- ^ Marker, A. F. H. (1972). "The use of acetone and methanol in the estimation of chlorophyll in the presence of phaeophytin". Freshwater Biology 2: 361. doi:10.1111/j.1365-2427.1972.tb00377.x
- ^ Jeffrey, S. W.; Shibata, Kazuo (February 1969). "Some Spectral Characteristics of Chlorophyll c from Tridacna crocea Zooxanthellae". Biological Bulletin (Marine Biological Laboratory) 136 (1): 54–62.doi:10.2307/1539668
- ^ Gilpin, Linda (21 March 2001). "Methods for analysis of benthic photosynthetic pigment". School of Life Sciences, Napier University. Retrieved 2010-07-17.
- ^ Woodward RB, Ayer WA, Beaton JM, Bickelhaupt F, Bonnett R, Buchschacher P, Closs GL, Dutler H, Hannah J, Hauck FP, Itô S, Langemann A, Le Goff E, Leimgruber W, Lwowski W, Sauer J, Valenta Z, Volz H. (July 1960). "The total synthesis of chlorophyll". Journal of the American Chemical Society 82 (14): 3800–3802. doi:10.1021/ja01499a093.
- ^ Fleming, Ian (14 October 1967). "Absolute Configuration and the Structure of Chlorophyll". Nature 216: 151–152. doi:10.1038/216151a0.
- ^ Robert Burns Woodward, William A. Ayer, John M. Beaton, Friedrich Bickelhaupt, Raymond Bonnett, Paul Buchschacher, Gerhard L. Closs, Hans Dutler, John Hannah, Fred P. Hauck, et al. (1990). "The total synthesis of chlorophyll a" (PDF, 0.5 MB). Tetrahedron 46 (22): 7599–7659. doi:10.1016/0040-4020(90)80003-Z.
- ^ http://www.scientificamerican.com/article.cfm?id=new-form-chlorophyll
- ^ http://www.newscientist.com/article/dn19338-infrared-chlorophyll-could-boost-solar-cells.html
- ^ Chen, M.; Schliep, M.; Willows, R. D.; Cai, Z. -L.; Neilan, B. A.; Scheer, H. (2010). "A Red-Shifted Chlorophyll". Science 329 (5997): 1318-1319. doi:10.1126/science.1191127. PMID 20724585.
- ^ Müller, Thomas; Ulrich, Markus; Ongania, Karl-Hans; Kräutler, Bernhard. (2007). "Colorless Tetrapyrrolic Chlorophyll Catabolites Found in Ripening Fruit Are Effective Antioxidants". Angewandte ChemieInternational Edition 46 (45): 8699–8702. doi:10.1002/anie.200703587. ISSN 1433-7851. PMID 17943948. PMC 2912502.
- ^ Gross, 1991
- ^ Meskauskiene R, Nater M, Goslings D, Kessler F, op den Camp R, Apel K. (23 October 2001). "FLU: A negative regulator of chlorophyll biosynthesis in Arabidopsisthaliana". Proceedings of the National Academy of Sciences 98 (22): 12826–12831. doi:10.1073/pnas.221252798. ISSN 0027-8424. PMID 11606728. PMC 60138.
- ^ Duble, Richard L.. "Iron Chlorosis in Turfgrass". Texas A&M University. Retrieved 2010-07-17.
- ^ http://www.adc.co.uk/Products/CCM-200_plus_Chlorophyll_Content_Meter
- ^ Adams, Jad (2004). Hideous absinthe : a history of the devil in a bottle. Madison, Wisconsin: University of Wisconsin Press. p. 22. ISBN 9780299200008.
- ^ US patent 5820916, Sagliano, Frank S. & Sagliano, Elizabeth A., "Method for growing and preserving wheatgrass nutrients and products thereof", issued 1998-10-13









Tidak ada komentar:
Posting Komentar